Журнал J. Solid State Chemistry
как распространитель ошибок и безграмотности
Группа бразильских ученых с привлечением немецкого коллеги, не имея достаточного опыта и не проработав имеющуюся литературу, взялась за изучение фазовых диаграмм систем с участием фторидов РЗЭ. И немедленно наломала дров, опубликовав результаты своих усилий в международных журналах (см. раздел «Ошибки при построении диаграмм», примеры 39 и 40).
Редакция журнала Material Research Bulletin без разговоров опубликовала письмо в редакцию [P.P. Fedorov. Comment on the paper “The phase diagram YF3 - GdF3” by D. Klimm, I. M. Ranieri, R. Bertram, and S. L. Baldochi. Materials Research Bulletin, 2012, v.47, p.2700]. Что же касается журнала Solid State Chemistry, то редколлегия грудью встала на защиту мундира. Переписку см. ниже.
Comment on the paper
“The phase diagram GdF3 - LuF3” by I. M. Ranieri, S. L. Baldochi, and D. Klimm
P. P. Fedorov
General Physics Institute, Moscow
I. M. Ranieri, S. L. Baldochi, and D. Klimm have presented their version of the GdF3 - LuF3 phase diagram in their paper [1] (Fig.1). They showed there (a) areas of the complete miscibility in liquid, (b) continuous solid solutions between low-temperature orthorhombic beta-polymorphous modifications of GdF3 and LuF3 and (c) limited solid solutions on the base of high-temperature alfa-YF3 and alfa - GdF3 modifications. These authors [1] also showed that two 3-phase equilibria occur:
eutectic
L = alfa-(Gd, Lu)F3 + alfa-(Lu,Gd)F3
and solid state (peritectoid)
beta-(Gd,Lu)F3 = alfa-(Gd, Lu)F3 + alfa-(Lu,Gd)F3
Unfortunately, their version of the diagram is incorrect.
The authors [1] were wrong when they stated that “the phase diagram of the system GdF3 - LuF3 is reported for the first time”.
Actually, the correct version of the GdF3 – LuF3 phase diagram (Fig. 2) has been reported long time ago [2-4] and published as a part of the extensive systematic studies of various RF3 – R’F3 systems (R and R’ – rare earth elements). The continuous solid solution with low temperature beta-YF3–type structure is under the equilibrium with the melt, and there are different 3-phase equilibria existing in the system:
eutectic
L = alfa-(Lu,Gd)F3 + beta - (Gd,Lu)F3
and peritectic
L + alfa- (Gd,Lu)F3 = beta- (Gd,Lu)F3.
The reason why results in [1] are erroneous is that authors [1] used “GdF3” samples contaminated with oxygen. Both significantly lower the polymorphic transformation temperature and significantly higher melting point support this conclusion. This effect was previously observed when Thoma [5] and Spedding [6] noted dramatic differences in phase transition temperatures (900 and 1077oC, respectively). This discrepancy was explained later, when Sobolev et al. [7, 8] showed that oxygen contamination was responsible for the above differences. An oxygen-containing admixture dissolves mainly in the high-temperature alpha- GdF3 modification, and thus expands the area of the existence of tysonite-type phase and causes superficial low-temperature polymorphism. In reality, the temperature effect at 900oC, observed by many authors, corresponds to the eutectoid alfa = beta +GdOF three-phase equilibrium, where alfa and beta are high- and low-temperature modifications of GdF3 [9] (for the further details and for the history of this problem please see [4]).
Authors [1] utilized inefficient fluorinating technique to rid their samples of oxygen. Also they did not carry out chemical analysis to determine the exact oxygen content in their specimen.
Comparison of “GdF3” thermogram from [1] and GdF3 - Gd2O3 phase diagram data [7] indicates that authors’ [1] sample(s) had about 1 mol. % Gd2O3 (Fig. 3). This amount of oxide impurity was sufficient to change the temperature of the observed polymorphous transformation of GdF3 by dozens degrees (oC), but it was not enough to observe the second phase in X-ray diffraction patterns. At the same time, according to LuF3 melting point and phase transformation temperatures, authors’ [1] LuF3 sample(s) was (were) sufficiently pure.
Therefore, “the phase diagram GdF3 - LuF3” proposed in [1] actually is a polythermal cross-section of the LuF3 - GdF3-Gd2O3 three-component system (for the probable scheme of the phase fields of its phase diagram please see Fig. 4).
Comparison of Fig. 2 and Fig. 4 data leads to the conclusion than even little oxygen contamination of GdF3 - LuF3 samples causes essential changes in the phase equilibria and moves the orthorhombic solid solution away from its equilibrium with the melt. The latter is in a complete agreement with the well-known observation that betta-YF3 orthorhombic crystals (Pnma space symmetry group) are very sensitive to the oxygen impurities in the batch, so the starting materials has to be thoroughly decontaminated from oxygen, and strong fluorinating atmosphere in the course of the crystal growth is a must [10].
References
1. I.M.Ranieri, S.L. Baldochi and D. Klimm, The phase diagram GdF3 - LuF3. J. Solid State Chemistry. 181 (2008) 1070-1074.
2. Sobolev B.P., Fedorov P.P., Ikrami D.D., Galkin A.K., and Sidorov V.S. Phase diagramms of the some LnF3 -Ln’F3 systems. // EUCHEM Conference on the chemistry of the rare earths. Helsinki, 1978. P. 134a-134b.
3. B.P.Sobolev, P.P.Fedorov, A.K.Galkin, V.S.Sidorov and D.D.Ikrami. Phase diagrams of binary systems formed by rare earth trifluorides.// Growth of Crystals. V.13, 1986. Ed. Givargizov. Consultants Вureau, New York and London.
4. Sobolev B.P. The Rare Earth Trifluorides. P.1. The High-Temperature Chemistry of the Rare Earth Trifluorides. Barcelona 2000.
5. Thoma R.E., and Brunton G.D. Equilibrium dimorphism of the lanthanide trifluorides. // Inorg.Chem. 1966. V.5. No.11. P.1937-1939
6. Spedding F.H., Beaudry B.J., Henderson D.C., and Moorman J. High-temperature enthalpies and related thermodynamic functions of the trifluorides of Sc, Ce, Sm, Eu, Gd, Tb, Dy, Er, Tm and Yb.//J. Chem. Phys.. 1974. V.60. No.4. P.1578-1588.
7. Sobolev B.P., Fedorov P.P., Steynberg D.B., Sinitsyn B.V., and Shakhkalamian G.S. On the problem of polymorphism and fusion of lanthanide trifluorides.I. The influence of oxygen on phase transition temperatures. J. Solid State Chemistry. 1976.V.17. ¹1/2. P.191-199.
8. Sobolev B.P., Fedorov P.P., Seiranyan K.B., Tkachenko N.L. On the problem of polymorphism and fusion of lanthanide trifluorides.II.
// J.Solid State Chemistry. 1976. V.17. No.1/2. P.201-212.
9. Greis O., Cader M.S.R. Polymorphism of high purity rare earth
trifluorides. // Therochimica Acta. 1985. V.87. P.145-150.
10. Fedorov P.P., and Osiko V.V. Crystal Growth of Fluorides. // In: Bulk Crystal Growth of Electronic, Optical and Optoelectronic Materials. Ed. P.Capper. Wiley Series in Materials for Electronic and Optoelectronic Applications. John Wiley & Son, Ltd. 2005. P. 339-356.
Figures :
Щелкните на рисунке для увеличения
Fig. 1. Erroneous version of the LuF3 – GdF3 system phase diagram [1].

Fig. 2. Correct version of the LuF3 – GdF3 system phase diagram [3].
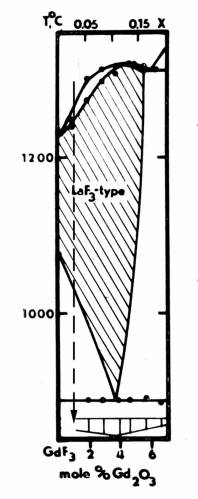
Fig. 3. The fragment of GdF3 – Gd2O3 phase diagram [ 7] with the marked position of “GdF3” sample studied in [1].

Fig. 4. Phase field scheme for the studied in [1] cross-section of the tri-component LuF3 – GdF3 – Gd2O3 system.
Решение Зура фон Лое не печатать письмо в журнал:
03.05.2012, 19:27, "JSSC" <jssc@elsevier.com>:
Ms. No.: JSSC-12-264
Title: Comment on the paper "The phase diagram GdF3 - LuF3" by I. M. Ranieri, S. L. Baldochi, and D. Klimm
Corresponding Author: Dr. Fedorov P. Pavel
Thank you for submitting your manuscript to the Journal of Solid State Chemistry. Your paper, referenced above, has been reviewed by experts in the field. Based on the comments of these reviewers, we regret to inform you that we are unable to accept your manuscript for publication in the Journal of Solid State Chemistry.
Neither reviewer feels that this letter should be published in its current form in JSSC and and both feel that the letter is too confrontational without a good reason for being so. Furthermore, they have some suggestions ranging form contacting Ranieri directly or publishing a full paper on the phase diagram.
The comments of the reviewers are included below in order for you to understand the basis for our decision, and we hope that their thoughtful comments will help you in your future studies.
While you may be disappointed by this decision, I would like to urge you to continue to consider the Journal of Solid State Chemistry for publication of future manuscripts.
Sincerely,
H.-C. zur Loye, Ph.D.
Associate Editor
Journal of Solid State Chemistry, Editorial Office
Elsevier
525 B Street, Suite 1900
San Diego, CA 92101-4495
USA
Fax: (619) 699-6700
E-mail: jssc@elsevier.com
Reviewers" comments:(if comments are not present please click on reviewer attachment link in EES)
Reviewer #1: The comment in review presents argumentation on why there is a disagreement between the phase diagram of the GdF3-LuF3 system published by Ranieri et al. (2008) and the earlier work of Sobolev et al. (references 2-4 in the comment).
The author of the comment criticizes Ranieri et al. for not citing the earlier work and points out that there are quite significant differences between the two diagrams. He also presents argumentation that this difference was caused by oxygen impurity in the GdF3, which shifted phase boundary lines.
The quality of writing in the comment is good, and the argument is logical, however, I do not like the confrontational tone of the comment and the very definitive statements such as "is incorrect", "used contaminated materials", "different from commonly accepted thermodynamic data" etc. W/o being able to analyze the materials used by Ranieri et al. this interpretation of the discrepancy in phase diagrams is only hypothetical and there might be other possible explanations, such as differences in instrument calibration routines, etc.
Neither of the three publications of Sobolev et al. were regular articles published in peer reviewed journals (one was an abstract in conference proceedings, the other two were included in book chapters), therefore I am not surprised that Ranieri et al. might have not had an access to the information contained in these publications. I am also not sure what criterion makes these "commonly accepted thermodynamic data."
In my opinion Ranieri et al. describe the sample preparation procedure in sufficient details so if one is interested in verifying the oxygen contamination hypothesis, they could attempt to follow it and obtain similar GdF3 materials, which could then be analyzed chemically. Reading another paper of the Ranieri group on LiF-GdF3 system (J. Alloys and Compounds, 2004, 379, 95-98) I can see that they were aware of the oxygen contamination possibility and knew how to look for its indications.
In conclusion, I do not recommend to publish the comment in Journal of Solid State Chemistry. For me the argumentation presented in the comment is not strong enough to publish a definitive statement of dismissal of the earlier paper. I think this comment could be turned into a regular article, if the author decided to do some experiments aimed at recreation of the oxygen contamination.
Reviewer #2: I have read with much interest your proposed manuscript, "Comment on the paper "The phase diagram ..."," which proposes to readdress the work of Ranieri et al on their proposed phase diagram of GdF3-LuF3 to reaffirm your, and others", phase diagram (&c) published variously as Sobolev et al. Several important points are made to place the work of Sobolev and others in a justified historical context and that make strong suggestions that Ranieri et al, in fact, observe a section of a wider, Gd2O3-containing, phase diagram. The arguments are made that propose to unify the prior art. In that regard, the work is significant to the field (though it is not the first time this has been covered in these pages; e.g. Sobolev, Federov et al; 1976 JSSC 17, 191.)
However, I was surprised, I feel quite strongly that the approach used is (or appears)... rather too confrontational, at worst, or too plain-speaking, at best. I certainly do not mind when previous work is called into doubt (indeed these are all the more interesting!) but in cases like these (and your manuscript is "Comment on the paper...", rather than, e.g. "The effect of oxygen ..."), one would assume that the Authors of the work being commented on have been contacted in the first instance and given the opportunity to respond privately or publicly. In the absence of any evidence of this in your rather thin cover letter and the absence of any attempt to state why, after four years since the publication of Ranieri et al, that this communication should be considered an important "Letter to the Editor" at JSSC, &c, I am left with assumptions. I have then concluded that the process you have undertaken does not put your work in its best light (irrespective of its content: you certainly make valid points). It is written, in my view, in a way that is not best-conducive to a profitable discourse and exchange between different groups.
I therefore urge you to detail fully your contact with Ranieri et al in the cover letter and (as I do not expect the scientific content to change any) to reword your manuscript with the average JSSC"s readers point-of-view in mind. I will suggest to the Editor that Ranieri et al be solicited for a Rebutal on your future comment, if there has been no contact with Ranieri et al.
To conclude, I agree that doubt is cast on phase diagram of Ranieri et al, through your highlighting of the, e.g. the effect of O in (particularly) GdF3. A compelling case is made that relies on work previously carried out, and indeed some of which has been published in these pages. However, I recommend a full revision to set a lighter tone to the work bearing in mind that it will be read by readers that have a lot less familiarity with this and related systems.
<Dear Dr. zur Loye,.docx>
Hanno zur Loye
David W. Robinson Palmetto Professor and
Associate Dean for Research
University of South Carolina
Department of Chemistry and Biochemistry
631 Sumter Street
Columbia, SC 29208
(803) 777-1934 Dean’s Office phone
(803) 777-6916 Chemistry Office phone
(803) 777-4532 Dean’s Office fax
(803) 777-8508 Chemistry Office fax
ZURLOYE@mailbox.sc.edu
http://www.chem.sc.edu
Director, Powder X-ray Diffraction Facility
http://www.chem.sc.edu/graduate/facilities_powder.asp
Associate Editor
Journal of Solid State Chemistry
JSSC@mailbox.sc.edu
http://www.sciencedirect.com/science/journal/00224596
Editor
Journal of Alloys and Compounds
JALCOM@mailbox.sc.edu
http://www.sciencedirect.com/science/journal/09258388
Energy Frontier Research Center
http://www.heterofoam.com
Ответ редактору:
From: Федоров Павел Павлович [mailto:ppfedorov@yandex.ru]
Sent: Thursday, July 05, 2012 7:15 PM
To: JSSC (ELS)
Subject: Re: JSSC-12-264: Final Decision
Dear Dr. zur Loye,
Thank you for your letter of May 3, 2012 regarding my JSSC-12-264 manuscript “Comment on the paper “The phase diagram GdF3-LuF3” by I. M. Ranieri, S. L. Baldochi and D. Klimm”. I strongly disagree with your decision to reject publication of my article and urge you to reconsider it. I protest your reviewers’ opinions, and I would like to tell you the following.
First, my paper is not written in a confrontational tone. The tone of my paper is quite adequate given the situation and, actually, it is softer than it could be; this is merely a rebuttal of Ranieri’s paper, which was published in your journal is a scandal.
The authors (Ranieri et al.) should have familiarized with the existing basic scientific literature on the topic of their publication prior to submitting their article to the Journal of Solid State Chemistry (JSSC). Once they obtained results, different from commonly accepted data, it was really necessary for them to analyze the reasons for such discrepancies. However, I would like to skip discussing the reasons why they hastily published their paper, for this will lead us away from the point of my present letter to you, namely, the problem that Ranieri’s paper has created for JSSC.
Unfortunately, this paper is not the only obviously erroneous publication in your journal. I even use the examples of wrong phase diagrams from JSSC in my graduate and undergraduate classes on phase diagrams in the special sections under the title “Errors in construction of phase diagrams”.
I fully understand that the appearance of papers with erroneous diagrams is a result of poorly qualified reviewers. Regretfully, this is exactly what happened in the first place when the reviewers of Ranieri’s paper did not exhibit sufficient competency, knowledge, attention and scrupulousness. A competent specialist should have realized at first glance that the “GdF3” thermogram in Ranieri’s publication did not correspond to a chemically pure substance. The reviewers could have had all the resources necessary for this conclusion (e.g., papers by Sobolev et al, JSSC, 1974 –references 7 and 8 in my manuscript), but they did not draw the correct conclusion.
Also I would like to address some reviewers’ comments.
Rebutting opinion of the reviewer #1, I would like to draw his/her attention that my criticism of Ranieri et al. in the submitted manuscript was not for non-citing the previous publications, it was primarily focused at the wrong interpretation of their own experimental data.
Reviewer #1 suggested that the temperature difference of more than 100oC stems out of a possibly poor calibrated thermocouple (“differences in instrument calibration routines”). This is not reasonable, for there is simply no way one can produce such poor experimental results unnoticed.
I consider that the reviewer’s #1 suggestion that only so-called peer-reviewed journal publications are acceptable sources for the scientific discussions is not reasonable and acceptable. Scientists use monographs (like refs. 2 and 3) and handbooks in their work frequently. These sources often contain time-tested experimental data, and Ranieri et al. could have benefited by reading earlier publications on the topic of their study. I have referenced two monographs specifically to emphasize that the data presented there had been accepted by the scientific community after their verification and confirmation. Once again, there was never criterion on what scientific data one may use in the discussion – from the original journal publications or from the monographs, - and if Journal of Solid State Chemistry (JSSC) introduces this kind of policy and adheres to it, it will be a new word in the area of research ethics.
Also if the reviewer #1 does not know how to distinguish commonly accepted data from something opposite in the certain field, then clearly a wrong reviewer was selected for this job. He/she could have used JANAF tables or some fundamental review papers, published by Elsevier, such as:
Greis O., Haschke J.M., 1982 In: Handbook on the Physics and Chemistry of Rare Earths, vol. 5. North-Holland, Amsterdam , pp. 387-460,
and
Kovacs, A., Konings, R.J.M., 2003. Thermodynamic properties of the lanthanide (III) halides. In: Gschneidner Jr., K.A., Bunzli, J.-C.G., Pecharsky, V.K. (Eds.), Handbook on the Physics and Chemistry of Rare Earths, vol. 33. North-Holland, Amsterdam, pp. 147-247.
Information, presented in the above sources, completely obviates the need “to do some experiments aimed at recreation of the oxygen contamination”. These experiments were done much earlier, and their results, in fact, were published in JSSC.
One of the major reasons, why Ranieri et al. obtained erroneous results, was their omission of the crucial step of melting the metal fluoride samples under a fluorinating atmosphere. This is a very well-known standard procedure of deep purification of synthesized fluorides to prevent oxygen contamination, and it was skipped by Ranieri et al. For more details of this protocol please see, for example:
C. E. Bamberger. In: Advances in Molten Salt Chemistry. V.3. Ed. J. Braunstein, G. Mamantov, G.P.Smith. Plenum Press: New-York-London. 1975. P. 177-248.
and
P.P. Fedorov, and V.V. Osiko. Crystal Growth of Fluorides. In: Bulk Crystal Growth of Electronic, Optical and Optoelectronic Materials. Ed. P. Capper. Wiley Series in Materials for Electronic and Optoelectronic Applications. John Wiley & Son, Ltd. 2005. P. 339-356.).
Countering opinion of the reviewer #2 and his/her suggestion to engage in the private communication with Ranieri et al. before sending my paper to JSSC, I would like to ask you why authors/scientists should turn to this concealed way of scientific discussion of publically-available data with their colleagues. I believe that if the journal publishes someone’s data and grants open access to them to the whole research community, then it should be opened to the same level of publicity in the discussion of the published materials. Please let me know if you consider otherwise.
In addition, I am also surprised why there is an issue with time limits when one should rethink already published results and consider straightening the scientific record (“… after four years since the publication of Ranieri et al.”). Writing my “Comment on the paper “The phase diagram GdF3-LuF3” by I. M. Ranieri, S. L. Baldochi and D. Klimm”, I designed it specifically for the “readers that have a lot less familiarity with this and related systems” in order to help them form a correct understanding of the subject matter.
I consider that at the present time there is no further necessity to study temperature of GdF3 phase transitions, and there is no need to publish an additional paper on this topic. This problem was successfully resolved by Spedding et al. about 40 years ago:
Spedding F.H.,Beaudry B.J.,Henderson D.C.,Moorman J. High-temperature enthalpies and related thermodynamic functions of the trifluorides of Sc,Ce,Sm,Eu,Gd,Tb,Dy, Er,Tm and Yb.//J. Chem. Phys.. 1974. V.60. No.4. P.1578-1588.
Spedding F.H., Henderson D.C. High-temperature heat contents and related thermodynamic functions of seven trifluorides of the rare earth Y, La, Pr, Nd, Gd, Ho, and Lu.// J.Chem.Phys. 1971. V.54.No.6. P.2476-2483.
Spedding’s data contradicted the earlier results of Thoma and Brunton (Thoma R.E., Brunton G.D. Equilibrium dimorphism of the lanthanide trifluorides. // Inorg.Chem. 1966. V.5. No.11. P.1937-1939) regarding existence or absence of phase transitions of the Rare Earth Element triflorides (including gadolinium) as well as the temperatures of these transformations. Differences between the results of the above research groups could not be reconciled, so the final resolution of this problem was offered by Sobolev et al. in:
Sobolev B.P., Fedorov P.P., Steynberg D.B., Sinitsyn B.V., Shakhkalanian G.S. On the problem of polymorphism and fusion of lanthanide trifluorides. I. Influence of oxygen on phase transition temperatures. // J.Solid State Chemistry. 1976. V.17. N 1/2. P.191-199.
DOI: 10.1016/0022-4596(76)90220-6.
and
Sobolev B.P., Fedorov P.P., Seiranyan K.B., Tkachenko N.L. On the problem of polymorphism and fusion of lanthanide trifluorides. II. Interaction of LnF3 with MF2 (M = Ca,Sr,Ba). Change in Structural type in the LnF3 series and thermal characteristics. //J.Solid State Chemistry. 1976. V.17. N 1/2. P.201-212.
DOI: 10.1016/0022-4596(76)90221-8
Sobolev et al. proved that Spedding and co-workers were right, and Thoma and Brunton were wrong. The reason for the differences between their results was oxygen contamination of the experimental samples by pyrohydrolysis. This interpretation has withstood the test of time, there is no ambivalence left, so, once again, it is absolutely unnecessary to carry out additional study and maintain the appearance of the existing “problem”.
At the same time, Thoma’s errors in trifluoride polymorphism seriously undermined his well-known results covering numerous NaF – RF3 systems. My colleagues and I have spent many years redoing and correcting his phase diagrams and eliminating multitude of his essential errors in his drawings. We wrote several papers in this area, including one review article (Fedorov P. P. Systems of alkali and rare-earth metal fluorides. // Russian J. Inorg. Chem. 1999. V. 44. N 11. P. 1703-1727). These articles have been published in Russian, but their English translations are easily available for readers. If you and your journal (JSSC) would consider detailed publication of experimental data for the full set of NaF – RF3 phase diagrams, I would think about this kind of offer, but this prospective paper unequivocally shall include discussion of Ranieri’s publications. The scientific community has increased its interest in this area of inorganic chemistry and material science because of modern problems related to nanotechnologies of optical materials, so there is no doubt that this article will be useful for journal readers.
Therefore, as stated above, I am not disappointed with your refusal to initiate a public discussion of the publicly available erroneous data. I am simply disgusted with your attitude lacking academic honesty and openness when your journal attempts to save its face after publishing erroneous results. I keep your journal, JSSC, responsible for spreading of false and erroneous data and subsequent pollution of scientific information. The position of your journal, which excludes discussion of already published materials, eliminates feedback system (which is mandatory for the normal development of science), and hence destroys science as a social phenomenon, is absolutely unacceptable. Please be advised that I reserve my right to expand this discussion of the scientific ethics to much broader circles of the scientific community.
Sincerely,
Prof. Dr. Pavel P. Fedorov
Technology of Nanomaterials for Photonics Laboratory, Laboratory Head
General Physics Institute, Russian Academy of Sciences, Moscow, Russia
Phone: +7-499-503-8292
Fax: +7-499-135-7744
E-mail: ppfedorov@yandex.ru
P. S. Also please be advised that my last name is Fedorov, not Pavel (this is my first name) as you have wrongfully mentioned it in your letter.
6. 07.2012
Ответ редактора на протест:
07.07.2012, 00:40, "CHEMISTRY JOURNAL, SOLID STATE C" <JSSC@mailbox.sc.edu>:
Dear Dr. Federov
As editors we have to rely on our reviewers in areas where we are not experts ourselves. In your case, both reviewers were in agreement. Hence my decision.
A letter, vs. a paper, is typically written to criticize existing work and, if done several years after the paper was published, comes across as vindictive rather than corrective. Might I therefore suggest that you submit a paper to JSSC on this phase diagram. In it you could demonstrate the correct phase diagram and point out that it should replace the previously published one.
sincerely
Hanno zur Loye
= = = =
On Jul 6, 2012, at 12:21 PM, JSSC (ELS) wrote:
Dear Prof. zur Loye,
Please see Dr. Federov’s email below concerning the rejection decision of his paper.
Best, Kathy

